Enhanced TDS
Identification & Functionality
- Chemical Name
- Industry
- Pharma & Nutraceuticals Functions
- Molecular formula
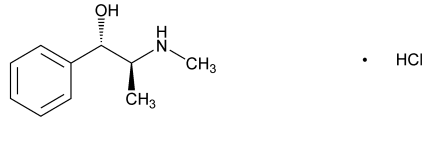
- (C₁₀H₁₅NO)·HCl
- Technologies
- Product Families
- Chemical Structure
Applications & Uses
Properties
- Physical Form
- Soluble In
- Appearance
- Fine white to off white crystals
- Odor
- Faint characteristic odor
- Soluble in
- Water, Ethanol (95%)
- Slightly Soluble in
- Chloroform
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 201.69 - - - Residual Solvents
Value Units Test Method / Conditions Acetone Content max. 1000 ppm Head space Gas chromatography Toluene Content max. 100 ppm Head space Gas chromatography - Specifications
Value Units Test Method / Conditions Melting Point (range between beginning and end of melting does not exceed 2°C) 182 - 186 °C - Loss on Drying (at 105°C, for 3 hrs) max. 0.5 %w/w - Specific Optical Rotation (at 24.5 - 25.5°C, 50 mg per ml in water) 61 - 62.5 ° - pH Value (50 mg/ml) 4.6 - 6 - - Residue on Ignition max. 0.1 %w/w - Assay (on dried basis) 99 - 101 %w/w Potentiometry - Impurities
Value Units Test Method / Conditions I-Ephedrine HCl Content (individual specified impurities) max. 0.1 %w/w High-Performance Liquid Chromatography Acetylephedrine Content (individual specified impurities) max. 0.1 %w/w High-Performance Liquid Chromatography Benzaldehyde Content (individual specified impurities) max. 0.1 %w/w High-Performance Liquid Chromatography Individual Unspecified Impurities Content max. 0.1 %w/w High-Performance Liquid Chromatography Total Unspecified Impurities Content max. 0.2 %w/w High-Performance Liquid Chromatography Total Impurities Content max. 0.4 %w/w High-Performance Liquid Chromatography
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
