Enhanced TDS
Identification & Functionality
- Chemical Name
- Industry
- Pharma & Nutraceuticals Functions
- Molecular formula
- C₈H₉NO₂
- Technologies
- Product Families
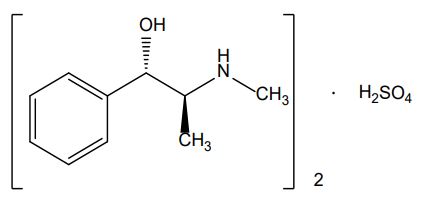
- Chemical Structure
Applications & Uses
- Markets
- Applications
- Manufacturing Technology
- Use Level
- 0.999 mg acetaminophen/mg (as is basis)
Properties
- Physical Form
- Soluble In
- Appearance
- White free flowing fine granules
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 151.16 - - - Microbiological Values
Value Units Test Method / Conditions Total Aerobic Bacteria Count max. 1000 cfu/g - Yeast and Mold Count max. 100 cfu/g LABPROC280 Escherichia coli None cfu/g - - Related Substances
Value Units Test Method / Conditions Impurity J Content (4-chloroacetanilide) max. 10 ppm - Impurity K Content (4-aminophenol) max. 50 ppm - Impurity F Content (4-nitrophenol) max. 0.05 % - Any Other Impurity Content max. 0.05 % - Total of Other Impurities Content max. 0.01 % - - Specifications
Value Units Test Method / Conditions Assay 87.5 - 92.5 % - Loss on Drying 1 - 2.4 % - Bulk Density 0.5 - 0.7 g/mL - Particle Size (retained on USS 20 mesh) max. 20 % - Particle Size (retained on USS 40 mesh) min. 40 % - Particle Size (retained on USS 60 mesh) min. 65 % - Particle Size (retained on USS 80 mesh) min. 75 % - Particle Size (on pan) max. 25 % -
Regulatory & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
